Best Practice ReviewChronic Cough - An Approach to Diagnosis and Management
Triya Chakravorty BA Oxon 1, Indranil Chakravorty PhD FRCP 21. University of Oxford, Medical Sciences Division 2. St Georges University of London, UK
ORCID ID https://orcid.org/0000-0003-2408-2234
Correspondence to:
Indranil.chakravorty@stgeorges.nhs.uk
ABSTRACT
Cough is a common manifestation of many respiratory conditions and mostly is non-specific on its own as a symptom of underlying disease. Most transient coughing episodes tend to settle within 2-3 weeks. Yet cough can herald more sinister disease such as malignancy or progressive respiratory conditions. In epidemiological surveys, cough persisting more than 8 weeks has been shown to have a significant impact on quality of life and is often difficult to diagnose and treat, taking weeks to months. There is consensus that a logical, evidence based, standardised approach is most likely to lead to an efficient diagnosis and provide the highest chance of effective resolution. This paper describes the current evidence and offers a best practice approach for primary care practitioners and general internists.
Keywords
Chronic cough, upper airway cough syndrome, post-nasal drip, gastro-oesophageal reflux
257 Reads
Cite as:
Chakravorty T, Chakravorty I. Chronic cough – An approach to diagnosis and management. The Physician
2020; vol 6 (issue 1) Epub 19.01.2020 (Pre-print v1) DOI: https://doi.org/10.13140/RG.2.2.30402.94400
Status - Under peer review - See comments from Reviewer 1 below (submitted 10.03.2020)
This article is published under post-publication peer review policy. If you wish to write a review of the article please email your comments including your full name, affiliation to
editor.thephysician@bapio.co.uk
of presentation accompanying this paper is available to view
Background
Cough is a defensive reflex that protects the airways in response to an inhaled foreign body or noxious and harmful environmental irritants. One of the commonest non-acute conditions presenting to a respiratory physician is a chronic, unexplained cough affecting approximately 12% of the population. It is associated with poor quality of life with psychological, social and physical consequences often leading to feeling fed-up and depressed. Patients typically complain of a dry irritating cough, driven by a strong urge to cough and usually associated with a discomfort located in the throat.
1 The severity and frequency of chronic cough is exceptionally difficult to measure or quantify. Traditionally, there is heterogeneity of chronic cough with the recognition of different types of cough which may be due to a variety of underlying aetiologies and therefore require specific approaches to treatment.
There are various definitions of when a cough requires further evaluation. In primary care, a cough lasting more than 3 weeks usually heralds a chest X-ray, a full blood count and if available, a spirometry. Provided there are no warning signs such as haemoptysis, weight loss or chest pain, most patients may receive a trial with an antibiotic, a bronchodilator, a short course of inhaled corticosteroids and sometimes a proton-pump inhibitor, before a specialist referral is warranted.
In some cases, investigations may reveal an ‘expected cause of cough’ such as asthma, gastro-oesophageal reflux, post-nasal drip or rhino-sinusitis but in such cases cough remains refractory to treatment. The American College of Chest Physicians in 2016, published results of a systematic review and guidance where any cough lasting more than 8 weeks without an identifiable cause from systematic investigations was defined by consensus, as ‘Unexplained Chronic Cough’
2.
Definition
Acute Cough (<3w)
Sub-acute cough (3-8w)
Chronic cough (>8w)
In population studies the most common cause of acute cough (< 3 weeks) were respiratory infections, (viral), exacerbations of asthma, chronic obstructive pulmonary disease (COPD) and pneumonia. Subacute cough (duration, 3-8 weeks) was most commonly associated with post-infectious cough, exacerbation of underlying diseases such as asthma, COPD, and upper airway cough syndrome (UACS). For chronic cough (> 8 weeks), common causes were UACS from rhino-sinus conditions, asthma, gastro-oesophageal reflux disease (GORD), non-asthmatic eosinophilic bronchitis, any combinations of these four conditions, and, less commonly, a variety of miscellaneous conditions including atopic cough.
3
Aetiology
There are a variety of aetiologies including (i) environmental causes such as cigarette smoke, air pollution (especially particulates), (ii) common respiratory conditions such as asthma, bronchitis and COPD, where the cough is typically related to the pathophysiology of the disease, (eg excessive airway mucus and inhalation of irritants), (iii) other causes include eosinophilic bronchitis, interstitial lung diseases, bronchiectasis, (iv) inadvertent side-effects of drugs (i.e. angiotensin-converting enzyme inhibitors) and (v) extra-pulmonary diseases, such as gastro-oesophageal reflux disease (GORD) and post-nasal drip secondary to rhinosinusitis. Furthermore, up to a quarter of patients may have multiple aetiologies combined.
As cough is ubiquitous in any population presenting to primary care or to general internists, it is most efficacious if clinicians work systematically towards a clear diagnosis, considering common before rare illnesses. In the past three decades, the diagnostic triad of asthma, GORD or rhinosinusitis in any combination has been suggested to be the likely cause of chronic cough. However, the vast majority of patients with these common conditions do not complain of persistent coughing or have features suggestive of cough hypersensitivity. Treatment of these conditions in patients with chronic cough may improve cough but rarely stops it completely. In some patients, however no clear cause can be identified, leading to the diagnosis of idiopathic cough.
Cough Hypersensitivity
Chronic cough is often associated with an increased response to tussive agents such as capsaicin, a phenomenon identified as cough hypersensitivity. Plastic changes in intrinsic and synaptic excitability in the brainstem, spine, or airway nerves can enhance the cough reflex, and can persist even after resolution of the initiating cough event. Structural and inflammatory airway mucosal changes in non-asthmatic chronic cough could represent the cause or the traumatic response to repetitive coughing. 4
Recent unravelling of the neurophysiology of cough, suggests that it is likely that neuronal dysfunction may be the primary cause of chronic cough. Indeed, evidence for such has been demonstrated by heightened cough responses to inhaled capsaicin in patients with chronic cough and asthma. In the presence of airway hyper-responsiveness, cough can be triggered by endogenous factors (asthma, GORD, post-nasal drip, even speaking and laughing) or exogenous factors (eg cold air, passive smoking, deodorants etc).
Neurophysiology
Activated sensory airway nerves transmit information via the vagus nerve to first synapse in the brainstem, which rapidly initiates the motor cough response 1. The cough reflex is thought to involve two main subtypes of sensory vagal afferent nerves. The first subtype is c-fibres; these form networks of unmyelinated nerves throughout the airways and are characteristically sensitive to capsaicin (chilli pepper extract) through activation of the transient receptor potential vanilloid type 1 (TRPV1) receptor and other irritant chemicals. They can also respond to other stimuli such as heat, acidity and inflammatory mediators. The second type, myelinated sub-epithelial Aδ fibres, are found in the proximal airways and respond to mechanical stimuli, osmolarity and acidity but do not typically express TRPV1, and are normally insensitive to capsaicin and inflammatory mediators. The morphology of these airway nerves has been delineated in human airway tissue and shows similarity to that seen in animal models (Fig 1).5 The transient receptor potential (TRP) ion channels are found abundantly in the airways, present in primary airway sensory neurons, and also in airway smooth muscle and epithelial cells. They have important functions in airway chemo-sensation and reflex control regarding temperature, osmolarity and oxidant stress. Reactive oxygen species that are induced by exposure to air pollutants can activate TRPV1 and TRPA1 to induce cough and could underlie air pollutant-induced cough. Increased expression of TRPV1 ion channels has been reported in airway epithelial nerves of patients with chronic cough.
P2X3 receptor antagonist, AF-219 ATP is known to activate and sensitise signal transmission at sensory sites including primary afferent neurons such as airway vagal afferent nerves via its P2X and P2Y receptors and P2X3-containing trimers. P2X3 antagonists have been shown to be active in many inflammatory and visceral pain models, by inhibiting inappropriate chronic signals and decreasing peripheral and central hypersensitivity.
Stimulating these airway nerves generates action potentials that synapse in the nucleus tractus solitarius (NTS) and paratrigeminal nucleus of the brainstem. These afferent nerves then activate complex neural networks, projecting to cortical and sub-cortical areas responsible for sensations of airway irritation and the urge to cough and ultimately, if the stimulus is sufficient, results in coughing via activation of spinal motor nerves to the diaphragm, intercostal muscles and larynx (Fig 2). Importantly, coughing can also be initiated voluntarily without any peripheral stimulus or precipitating sensations, and in some cases voluntarily suppressed. 6 Thus, the potential drivers of excessive cough could originate either in the peripheral nerves or central nervous system, including the brainstem.
Hypersensitive or hyper-responsive cough?
Recent consensus suggests that ‘Cough Hypersensitivity Syndrome’ (CHS) be used to describe patients with chronic cough.7 However, evidence from experimentally evoked cough suggests that the neuronal pathways exhibit hyper-responsiveness rather than hypersensitivity. Patients complain of an inability to stop coughing and quality of life is most severely impacted by the length and severity of coughing bouts.
The concept of CHS is that there is a stage of peripheral sensitisation induced by inflammatory factors setting up the scene for a central component that can be visualised by functional magnetic resonance imaging (fMRI).8 One of the potential mechanisms underlying CHS is that this may be triggered by an inflammatory process that impacts on the nerve endings that increases the sensitivity of these nerves leading to peripheral sensitisation. There is already some evidence that in idiopathic cough, there is inflammation measured in terms of inflammatory cells such as mast cells and of inflammatory cytokines in the upper and lower airways. In addition, in conditions where chronic cough could be a predominant symptom such as asthma, COPD and pulmonary fibrosis, there is a characteristic inflammatory changes for that disease that could interact with cough sensory nerves.
Measuring Cough Hypersensitivity
Measurement of cough hypersensitivity with citric acid or capsaicin indicate that patients with chronic cough usually demonstrate an excessive cough response to inhaled tussigens, with correlation obtained between the level of the neuroinflammatory mediators and the degree of the cough tussive response, supporting the value of cough provocation tests in the diagnosis of CHS. The larynx is an area where the cough hypersensitivity may originate, manifest as inappropriate vocal cord adduction (associated with difficulty in breathing and dyspnoea), globus pharyngeus, impaired phonation (associated with paradoxical vocal fold motion) and muscle tension. This hypersensitivity has been described in athletes who develop cough and dyspnoea during usually with intense exercise.
Imaging of the brain using fMRI physiological sensory circuits, Mazzone et al have shown that sensory hypersensitivity is represented by both an enhanced activity of the brain regions encoding sensation as well as abnormal responses in brain circuits that usually have descending control on primary afferent processing 8. Patients with chronic cough demonstrating CHS, had increased activity in the midbrain regions that are involved in nociceptive control.
Cough associated with respiratory conditions
Cough is often dissociated from other symptoms usually attributable to asthma such as wheeze and shortness of breath. In COPD, cough is reported in 70% of patients, and many consider it to be extremely severe contributing to impaired quality of life. Current smokers with COPD tend to have the highest cough rates, almost double that of COPD + ex-smokers or healthy smokers.
Investigations
Assessment of cough may include a simple visual analogue scale (VAS), cough symptom score, quality of life questionnaire, cough frequency monitoring, and cough provocation test. These tests are used to monitor disease status and treatment efficacy.
In the VAS scoring system, patients mark a point on a straight line corresponding to their perception of the severity of cough. The score ranges from 0–10 cm (0–100 mm), with 0 representing minimal severity and 10 representing extreme severity. Compared with the cough symptoms score, the intervals between grades with the VAS are smaller, which is helpful for longitudinal comparison before and after treatment.
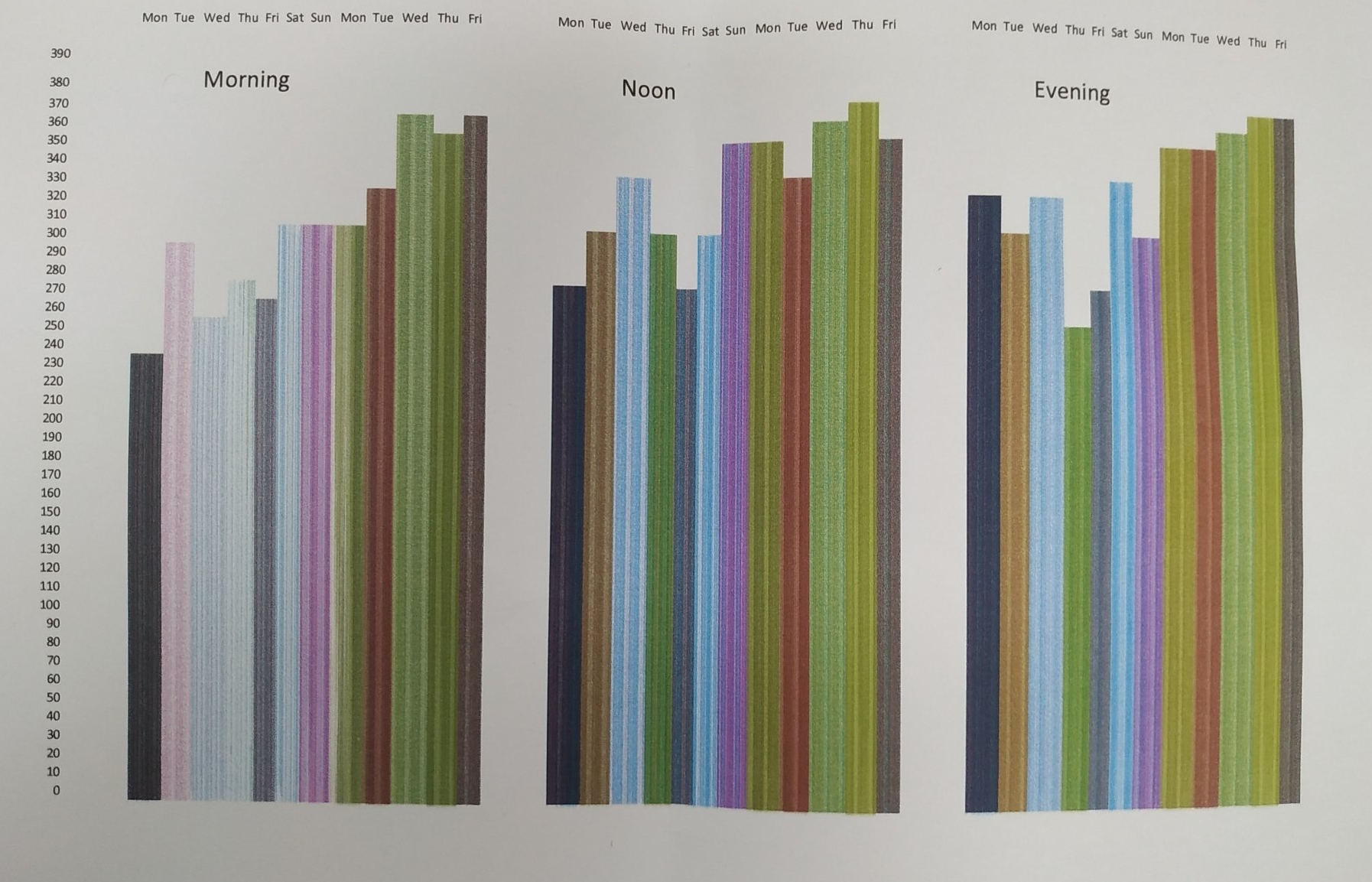
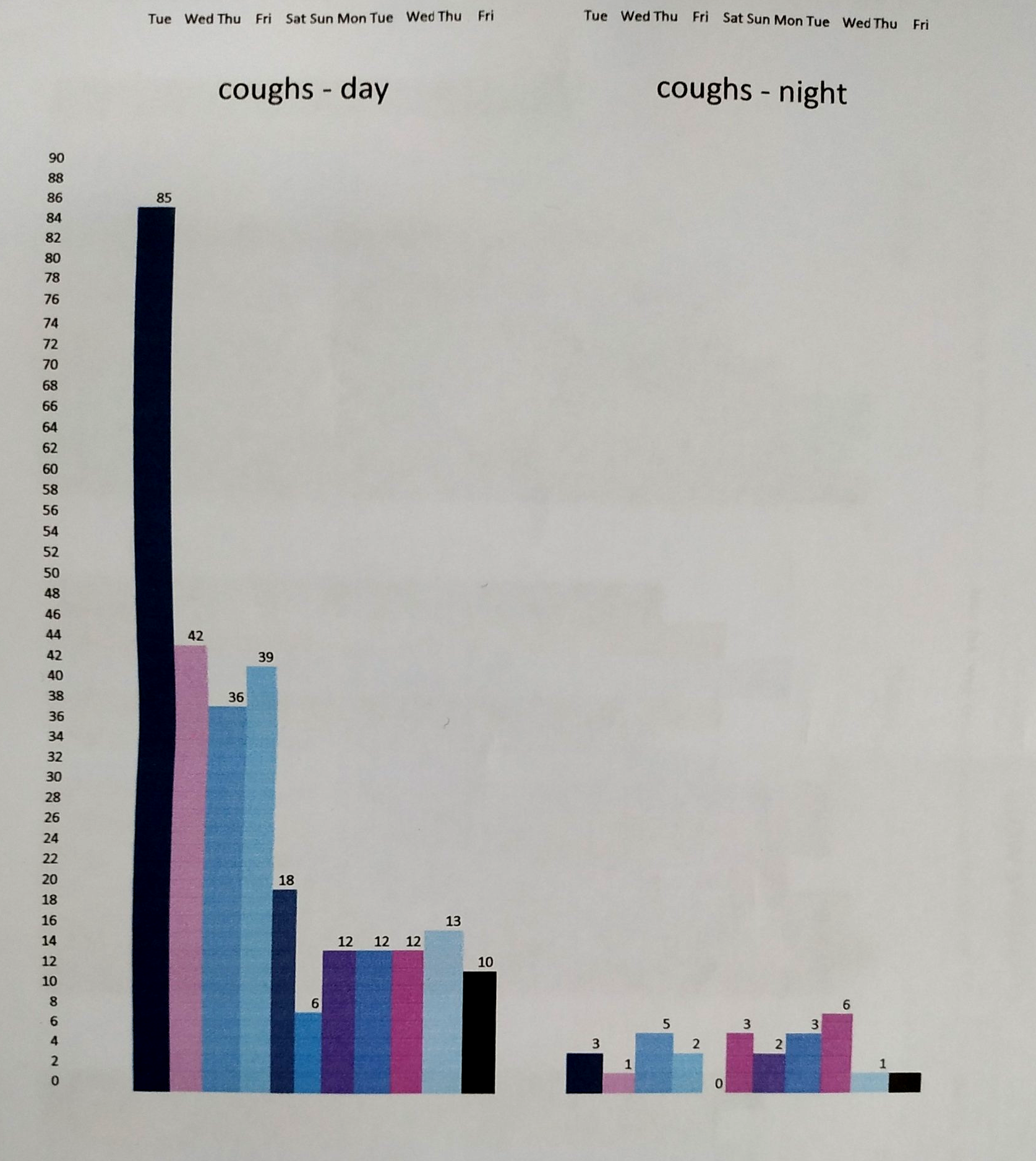
The Coughing Score is a quantitative scoring system used to assess the severity of cough and efficacy of treatment. Daytime and night-time scoring is done, however it may be difficult to discriminate between grades 9. Appreciation of the impact of cough on health-related quality of life has led to the development of three validated, cough-specific, health-related quality-of-life questionnaires that assess cough severity: Leicester Cough Questionnaire (LCQ)10, Cough-specific Quality of Life Questionnaire (CQLQ)11, and Chronic Cough Impact Questionnaire (CCIQ)12. These tools capture additional information not measured with objective tools and can be used to assess therapy. They should be used in conjunction with other cough severity measures such as cough frequency monitors to obtain a more complete assessment of cough severity.13
Investigation for causes of chronic cough are commonly chest radiography, bronchial hyper-responsiveness (BHR) and sinus imaging. Specialised investigations of GORD by using oesophageal pH probe monitoring, a chest CT scan or induced sputum (for eosinophilic bronchitis) are uncommon. Fractional exhaled nitric oxide (FeNO) and maximum mid-expiratory flow (MMEF) might have value as negative parameters for differentiating cough variant asthma (CVA) from chronic cough. 14
Differential Diagnosis
The common causes of chronic cough are as follows:
• Cough variant asthma (46%)
• Upper airway cough syndrome/postnasal drip syndrome (32%)
• Eosinophilic bronchitis (9%
• Gastroesophageal reflux-related chronic cough (9%)
• Postinfectious cough (6%)
• Angiotensin-converting enzyme inhibitors-induced cough (5%) {Yu et al}
In addition to respiratory disease, cough may be a manifestation of cardiovascular, autonomic or neurological disease.
Chronic cough is difficult to manage, and many patients self-medicate with ‘over the counter’ cough therapies despite lack of evidence supporting their efficacy. A survey of of chronic cough in general practice estimated that 87% of patients could have been managed solely in primary care using a simple guide and that most cases of chronic cough referred to secondary care could be managed with a simple systematic approach. This indicates that efforts need to be made to improve the management of such patients in general practice.
Stage I
Most patients who develop a cough will see their general practitioner first, who will exclude any obvious cause of cough and will most likely order a chest radiograph to exclude any gross pathology in the airways. Asthma and GORD are usually considered and may be excluded with a trial of inhaled corticosteroid therapy and proton pump inhibitor.
Stage II
The next stage is a referral to the hospital respiratory clinic, usually to a chest specialist, although referrals can be made to an otorhinolaryngologist for exclusion of upper airway nasal/laryngeal causes, or to a gastroenterology specialist for exclusion of GORD. Under hospital care, certain additional investigations are usually organised including a lung function test, bronchial hyper-responsiveness, a CT scan of sinuses and thorax and a bronchoscopy may be available.
Stage III
When all known causes of chronic cough have been excluded, and cough is still persistent, the skills of a highly specialised chronic cough may be required. These clinics should provide a multidisciplinary approach to diagnosis and management, and should have access to various facilities for assessment of oesophageal function, nasal and laryngeal hypersensitivity measurements, sleep studies, computed tomography of upper airways and lungs, and have access to otorhinolaryngology facilities such as the visualisation and assessment of the postnasal space and larynx and nasal passages. A speech and language therapist should be part of the team. An important role for specialist cough centres is in educating and training primary and secondary care in the management of chronic cough.
Management of Chronic Cough
Non-pharmacological Measures
This non-pharmacological approach consists of education, cough suppression strategies, vocal hygiene training, and psychoeducational counselling. This has the goal of improving voluntary control over the cough, by teaching patients to identify the causes and sensations that precipitate the cough and to replace the cough response with another response such as a breathing or swallowing exercise, and to alter behaviour that contribute to laryngeal irritation. This method likely acts on both peripheral and central parts of the cough pathway.
(a) Cough control measures such as Buteyko breathing control
techniques 14 have been shown to improve quality of life and inhaled corticosteroid use by reducing the perception of breathlessness. Although the ‘physiology’ suggested is of reducing shear stress that leads to mast cell activation 15, several studies have failed to show any change in physiological measures of asthma. 16
(b) Yoga- Tai Chi
17 18 have been found to be more effective than usual care in COPD with clinically meaningful improvements in 6-min walk distance, lung function and health-related quality of life. They have also been found to be comparable to pulmonary rehabilitation interventions in improving breathing control, reduced perception of breathlessness and cough. Mindfulness techniques have been shown to improve perceptions of breathlessness and symptoms in patients with chronic lung disease and may have a role in the management of cough. 19
(c) Behavioural - Speech therapy
may be under utilised in practice and could lead to improvement of otherwise recalcitrant cough.20 A multi-dimensional speech pathology treatment programme (used to treat hyper-functional voice disorders and paradoxical vocal fold movement) included education, vocal hygiene training, cough suppression strategies and psycho-educational counselling. Participants demonstrated a significant reduction in cough, breathing, voice and upper airway symptoms following intervention. 21 22. However the availability of specialist behavioural-speech therapy services is usually restricted to tertiary units and the usual waiting time is over 2 years in the UK.
Pharmacological Measures
(a) Macrolide antibiotic
treatment has beneficial effects on lung function in non-asthmatic, productive, chronic cough patients with normal chest X-ray findings. The improvement of chronic rhinosinusitis may have some role in the lung condition.23 Patients demonstrated neutrophilic or pauci-granulocytic airway inflammation, whereas subjects with eosinophilic airways inflammation do not appear to respond symptomatically.24 The agreed consensus suggests at least a prolonged therapy of up to 3 months to assess benefit.
(b) Proton-pump inhibitors
(PPI) – PPIs are ineffective as single agents in the absence of significant acid GORD. A cohort of GORD patients may present with more proximal reflux, non-acid reflux, and gas reflux, and get better efficacy with neuromodulators (gabapentin or baclofen) used as an add-on therapy with a proton-pump inhibitor. 25 Although gabapentin and morphine exhibit positive effects on cough-related quality of life, only gabapentin is currently supported as a treatment recommendation.
(c) Inhaled corticosteroids
(ICS) were found to be ineffective for chronic cough except in the presence of asthma or eosinophilic bronchitis. 2
(d) Neuromodulatory therapies
are believed to act on the enhanced neural sensitization that is a key component of unexplained cough. Each of the centrally acting neuromodulators (amitriptyline, pregabalin and gabapentin) have been shown to have positive effects on cough-specific quality of life. Adverse effects can be significant and limit the maximum tolerable dose of these agents. Recommendations suggest that reassessment of the risk-benefit profile be performed at 6 months.
(e) Codeine & Morphine
could be used when all other therapeutic options have failed to improve cough and there was close follow-up at 1 week and then monthly.
New targets for chronic cough
(i) Transient Receptor Potential ion channel antagonists - There are TRPV1 and TRPA1 channel blockers in development but it is currently unclear whether they are of benefit in management of chronic cough.
(ii) AF-219 is a P2X3 receptor antagonist - holds promise as a potentially new neuromodulator drug for chronic idiopathic cough, and also for chronic cough associated with chronic respiratory diseases such as asthma, COPD and pulmonary fibrosis. 26 However all patients had taste disturbances (hypogeusia or dysgeusia)
(iii) Central neuromodulators - Central targets that selectively disrupt specific encoding mechanisms of cough may present new therapeutic approaches such as the acid-sensing Ion channels (ASIC). N-methyl-d-aspartate (NMDA) receptors are involved in these acid-evoked reflexes. Memantine, an NMDA channel blocker, has been shown to suppress citric-acid induced cough. 8
Conclusion
Cough is a common presentation to primary care and to internists and may have a significant impact on quality of life and often lead to social and psychological consequences. Once, acute or sinister causes of cough have been excluded in primary or secondary care, a systematic approach is warranted. Even in chronic cough (>8 weeks), the triad of cough variant asthma, rhinosinusitis – postnasal drip and GORD may be the cause of majority of presentations and often in combination. Therefore, a combined therapeutic approach with objective cough intensity and impact measures (Leicester Cough Questionnaire + cough counters) are beneficial. If these commoner conditions have been excluded, a specialist multi-disciplinary approach is recommended. This will involve specialised investigations, provocation testing, and a comprehensive approach with education, physiotherapy, cough suppression and neuro-modulators. Careful and close monitoring of the benefits of this treatment is essential with objective measures and psychological support may also be needed. There are newer agents being tested approaching the cough reflex from a neuro-biology and inflammatory aspects.
References
4. Chung, K. F. & Pavord, I. D. Prevalence, pathogenesis, and causes of chronic cough. The Lancet 371, 1364–1374 (2008).
5. West, P. W., Canning, B. J., Merlo-Pich, E., Woodcock, A. A. & Smith, J. A. Morphologic Characterization of Nerves in Whole-Mount Airway Biopsies. Am. J. Respir. Crit. Care Med. 192, 30–39 (2015).
6. Young, E. C. et al. The effect of mindfulness meditation on cough reflex sensitivity. Thorax 64, 993–998 (2009).
7. Morice, A. H. Chronic cough hypersensitivity syndrome. Cough 9, 14 (2013).
8. Mazzone, S. B., Chung, K. F. & McGarvey, L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir. Med. 6, 636–646 (2018).
9. Chung, K. F. Measurement of cough. Respir. Physiol. Neurobiol. 152, 329–339 (2006).
10. Ward, N. The Leicester Cough Questionnaire. J. Physiother. 62, (2015).
11. French, C. T., Irwin, R. S., Fletcher, K. E. & Adams, T. M. Evaluation of a Cough-Specific Quality-of-Life Questionnaire. CHEST 121, 1123–1131 (2002).
12. Baiardini, I. et al. A new tool to assess and monitor the burden of chronic cough on quality of life: Chronic Cough Impact Questionnaire. Allergy 60, 482–488 (2005).
13. Brignall, K., Jayaraman, B. & Birring, S. S. Quality of life and psychosocial aspects of cough. Lung 186 Suppl 1, S55-58 (2008).
14. Cowie, R. L., Conley, D. P., Underwood, M. F. & Reader, P. G. A randomised controlled trial of the Buteyko technique as an adjunct to conventional management of asthma. Respir. Med. 102, 726–732 (2008).
15. Chowdhary, R. et al. Relationship of flow and cross-sectional area to frictional stress in airway models of asthma. J. Asthma Off. J. Assoc. Care Asthma 36, 419–426 (1999).
16. Bruton, A. & Lewith, G. T. The Buteyko breathing technique for asthma: a review. Complement. Ther. Med. 13, 41–46 (2005).
17. Wu, W. et al. Effects of Tai Chi on exercise capacity and health-related quality of life in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 1253–1263 (2014).
18. Ratarasarn, K. & Kundu, A. Yoga and Tai Chi: a mind–body approach in managing respiratory symptoms in obstructive lung diseases. Curr. Opin. Pulm. Med. Publish Ahead of Print, (2020).
19. Perkins-Porras, L. et al. Feasibility study to assess the effect of a brief mindfulness intervention for patients with chronic obstructive pulmonary disease: A randomised controlled trial. Chron. Respir. Dis. 15, 400–410 (2018).
20. Treatment of Chronic Cough: Single-Institution Experience Utilising Behavioral Therapy - Resha S. Soni, Barbara Ebersole, Nausheen Jamal, 2017. https://journals.sagepub.com/doi/10.1177/0194599816675299.
21. Gibson, P. G. & Vertigan, A. E. Speech pathology for chronic cough: a new approach. Pulm. Pharmacol. Ther. 22, 159–162 (2009).
22. Murry, T. & Sapienza, C. The Role of Voice Therapy in the Management of Paradoxical Vocal Fold Motion, Chronic Cough, and Laryngospasm. Otolaryngol. Clin. North Am. 43, 73–83 (2010).
23. Kariya, S. et al. Long-term treatment with clarithromycin and carbocisteine improves lung function in chronic cough patients with chronic rhinosinusitis. Am. J. Otolaryngol. 41, 102315 (2020).
24. Martin, M. J. et al. Idiopathic chronic productive cough and response to open-label macrolide therapy: An observational study. Respirol. Carlton Vic 24, 558–565 (2019).
25. Chen, L.-C. et al. Diagnostic value of FeNO and MMEF for predicting cough variant asthma in chronic cough patients with or without allergic rhinitis. J. Asthma Off. J. Assoc. Care Asthma 1–8 (2019) doi:10.1080/02770903.2019.1694035.
26. Abdulqawi, R. et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. The Lancet
385, 1198–1205 (2015).
Fig 1: Structure of human airway nerves. A – thin unmyelinated c-fibres located near the -epithelial membrane; B – sub-epithelial ¬myelinated Aδ fibres. Reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society. 1
Fig 2.Schematic diagram representing the cough reflex. Vagal afferents transmit stimuli from the airways to the nucleus tractus solitarius (nTS) and paratrigeminal nucleus (Para V) in the brainstem. Neuronal signals are then transmitted to the somatosensory cortex via the thalamus causing throat irritation and urge to cough. These sensations, if great enough, lead to cough via activation of spinal motor neurons. 1
Approach to Cough- schematic
PEER REVIEW Chakravorty T, Chakravorty I. Chronic cough – An approach to diagnosis and management. The Physician 2020; vol 6 (issue 1) Epub 19.01.2020
Reviewer 1
(submitted 10.03.20) Dr Ananthakrishnan Raghuram MD FRCP MScConsultant Respiratory Physician, Cheltenham General HospitalHead of Postgraduate School of Medicine, Health Education England, working in the southwest; Linacre Fellow RCP London
Consultant Respiratory Physician, Cheltenham General Hospital
Head of Postgraduate School of Medicine, Health Education England, working in the southwest;
Linacre Fellow RCP London
- This is a good article on cough and describes the definitions, aetiology and management of cough
- It would have been helpful to describe the various phenotypes of cough including that secondary to nicotine use
- The BTS guidance in 2006 by Morice et al is worth referencing as is the ACCP guidance Pratter et al 2006
- The latest guidance is from the ERS and is well worth referencing - Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2019; in press (https://doi.org/10.1183/13993003.01136-2019)
- CT scanning for chronic cough in the absence of red flags needs to be considered carefully given the risk of radiation
- The role of low dose morphine in chronic cough could have been explored in more detail - See reference Sheklly et al 2017
REVIEW
Assessing Cardiovascular Safety in the Development of New Drugs for Type 2 Diabetes Mellitus
J. Rick Turner, PhD
J.Rick Turner, PhD, is Senior Director, Clinical Communications, Quintiles. He is a clinical triallist, author, and editor who has published 130 peer-reviewed papers and articles in professional journals, and published 14 books. He has testified before two US Food and Drug Administration Advisory Committees with regard to the cardiovascular safety of antidiabetic drugs for Type 2 Diabetes Mellitus. Email: rick.turner@quintiles.com
Paul Strumph, MD
Paul Strumph, MD, is Vice President, Therapeutic Strategy Lead, Cardiovascular and Metabolic Therapeutic Delivery Unit, Quintiles, and the Head of their Diabetes Center of Excellence. He is a Diplomate in Internal Medicine, Paediatrics, Adult Endocrinology, Diabetes and Metabolism, and Paediatric Endocrinology. He is an author of more than a dozen articles in scientific journals and magazines, including Nature, Clinical Paediatrics, the Journal of Clinical Endocrinology and Metabolism, and Diabetes Care.
Email: paul.strumph@quintiles.com
cite:
Turner JR, Strumph P. Assessing Cardiovascular Safety in the Development of New Drugs for Type 2 Diabetes Mellitus.
The Physician
2012 1(1): 34-37
Along with obesity and cardiovascular disease, type 2 diabetes mellitus (T2DM) is a global public health concern of staggering proportions.1 The International Diabetes Federation (IDF) recently observed that, in 2011, there were 366 million people with diabetes, a figure expected to rise to 552 million by 2030.2 Taking the United States as one example of a large Western country, the 2011 National Diabetes Fact Sheet3 stated that 25.8 million children and adults in the United States — 8.3% of the population — have diabetes, with 18.8 million being diagnosed and 7.0 million being
undiagnosed. Another 79 million people have prediabetes. (It should be noted that the Fact Sheet used both fasting glucose and A1c levels to derive estimates for undiagnosed diabetes and prediabetes: these tests were chosen since they are most frequently used in clinical practice in that country.) While these numbers are of considerable
magnitude, the IDF also observed that most people with diabetes live in low- and middle-income countries, and it is these countries that will see the greatest increase between now and 2030.2
While preventive measures such as eating an appropriate diet and engaging in regular physical activity have been shown to be effective in preventing the progression of prediabetes to diabetes in some people,4,5 many individuals require medications to treat T2DM. As the European Medicines Agency (EMA) has noted, “Glucose control in type 2 diabetes deteriorates progressively over time, and, after failure of diet and exercise alone, needs on average a new intervention with glucose lowering agents every 3-4 years in order to obtain/retain good control.”6 The latter part of the quote makes clear that, while there are good drugs currently on the market, there is an ongoing need for additional drugs
to be developed. This paper therefore reviews recent guidance by both the EMA and the United States (US) Food and Drug Administration (FDA) concerning the development of such drugs, and specifically the cardiovascular safety of these compounds. The authors believe that an understanding of the requirements and challenges involved in bringing all drugs to market is beneficial not only for those actively involved in life-cycle drug development but also for prescribing physicians and health professionals who dispense and administer pharmaceutical medicines to patients. Clinical research informs clinical practice and evidence-based medicine, and practising physicians can benefit from having sufficient knowledge about clinical research and drug development programmes to understand their role in placing new drugs within their treatment armamentaria and generating the evidence contained in treatment practice guidelines.7
Assessing Cardiovascular Safety
Since 2008, new regulatory landscapes have emerged in the US8 and the European Union (EU)6 addressing the prospective exclusion of unacceptable cardiovascular risk in the development of new antidiabetic drugs for the treatment of T2DM. These are reviewed in turn. US Regulatory Landscape In February 2008 the FDA released a general draft guidance entitled “Diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention.”9
Its contents did not cover cardiovascular safety, but commented as follows:
A premarketing recommendation to demonstrate macrovascular risk reduction in the absence of a signal for an adverse cardiovascular effect may delay availability of many effective antidiabetic drugs for a progressive disease that often requires multiple drug therapy. A reasonable approach may be to conduct long-term cardiovascular studies post approval in an established time frame…This approach is beyond the scope of this guidance. In July 2008, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee held a meeting related to cardiovascular safety assessment,
the end result of which was a 14-2 yes/no vote that, even for antidiabetic drugs without a concerning cardiovascular safety signal during Phase II/ Phase III development, there should be a requirement to conduct a longterm cardiovascular trial or to provide other equivalent evidence to rule out an unacceptable cardiovascular risk.10 Subsequently, in December 2008, the FDA issued a guidance addressing this issue entitled “Guidance for Industry. Diabetes Mellitus:
Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes” in final format.8, 11, 12. The guidance requires sponsors to provide compelling evidence that new agents to treat T2DM are not associated with an unacceptable increase in cardiovascular risk, operationalised as an unacceptable increase in the number of adverse clinical cardiovascular events. Clinical endpoints of interest include, but are not limited to, non-fatal myocardial
infarction, non-fatal stroke, and cardiovascular mortality (events which comprise the Major Adverse Cardiovascular Events [MACE] composite endpoint), acute coronary syndrome, and urgent revascularisation procedures. A composite endpoint can be advantageous in circumstances in which the number of individual events can be too low to meaningfully compare those occurring in the test drug treatment group with those occurring in the comparator treatment group. The guidance also makes clear that endpoints require independent adjudication.13
Additional changes to development programmes going forward include the length of trials to be conducted and the nature of the subject population employed. Larger and longer late Phase II trials are called for, as are larger and longer Phase III trials that include subjects at high risk for cardiovascular events. The approach to excluding unacceptable risk can be represented by a three-component model incorporating clinical science (clinical judgments concerning absolute and relative risks), regulatory science (benefit-risk judgments at the public health level and choice of thresholds of regulatory interest), and statistical science (determining whether or not regulatory thresholds have been breached).14 On this occasion, the regulatory thresholds of interest are 1.8 and 1.3, which are to be discharged sequentially: the nature of these values is explained shortly.
Upon completion of a planned preapproval clinical development programme, a meta-analysis exploring the investigational drug’s MACE liability is conducted by incorporating data from Phase II and Phase III trials. Risk is operationalised in terms of a relative risk ratio, with the number of MACE composite endpoint events in the drug treatment group as the numerator and the number of such events in the control group as the denominator. This calculation yields the risk ratio point estimate. Two-sided 95% confidence intervals (CIs) are then placed around this point estimate. Attention falls on the upper CI limit, with a value of 1.8 or greater attracting regulatory concern (see Turner15 for a more detailed explanation of this statistical approach). Three scenarios are described in the guidance:
• If the upper limit of the CI is equal to or greater than 1.8, the drug would be deemed to have an unacceptable risk. In this case, “an additional single, large safety trial should be conducted that alone, or added to other trials, would be able to satisfy this upper bound before NDA/BLA submission.”8
• If the upper bound is equal to or greater than 1.3 and also less than 1.8, and the overall risk-benefit analysis presented at submission supports marketing approval, “a postmarketing trial generally will be necessary to definitively show that the upper bound of the two-sided 95 percent confidence interval for the estimated risk ratio is less than 1.3.”8 Having discharged the 1.8 value at the time of marketing application, the 1.3 value is then to be discharged in a second step. The postmarketing trial referred to in the previous bullet is a large-scale cardiovascular outcomes safety trial focusing on
MACE outcomes:
this trial will be discussed in the next section.
It should be noted that, while unlikely in practical terms, it is possible that the 1.3 value (and hence also the 1.8 value) could be discharged by the meta-analysis of premarketing data. The guidance addresses such a scenario as follows:
• If the upper limit is less than 1.3 and the overall risk-benefit analysis presented at submission supports marketing approval, “a postmarketing cardiovascular trial generally may not be necessary.”8 A review by Joffe et al.16 provides a more detailed review of the guidance’s content and consequences, and is recommended to readers. As these authors noted, “The new approach to developing medications for the treatment of type 2 diabetes will lead to evaluation in patients more representative of those who will use these therapies, if approved, and will help healthcare providers make informed decisions when choosing a medication within the growing treatment armamentarium for type 2 diabetes.” Their comment further emphasises the benefits to prescribing physicians of a good knowledge of how new drugs for T2DM
are developed and approved.
EU Regulatory Landscape
The EMA draft guideline makes it clear that two approaches prospectively excluding unacceptable cardiovascular risk are conceivable. The first is a meta-analytic approach, similar in spirit to the one discussed in the FDA guidance. With regard to the second, the guidance comments as follows: As an alternate approach or when there is suspicion of an adverse CV
signal (from the database), a specific long-term controlled outcome study with at least 18-24 months follow-up (depending on the characteristic of a drug and baseline risk of the studied population) would be expected as part of the clinical development program of new glucose lowering agents at the time of submission of the MAA.6
With two notable exceptions, the approaches in the FDA and EMA documents are comparable. These salient difference are: 1 there are no explicit thresholds of regulatory concern corresponding to the values of 1.8 and 1.3 as presented in the FDA document with regard to a meta-analysis of data from Phase II and Phase III trials; and 2 the EMA wishes to be fully satisfied at the time of granting marketing approval that there are no cardiovascular safety liabilities. This contrasts with the FDA’s approach of sponsors satisfying a regulatory threshold of 1.8 at the time of granting marketing approval, and subsequently using postmarketing data to satisfy the 1.3 threshold.
Large-scale Cardiovascular Outcomes Trials
The literature provides various examples of studies underway. In a recent editorial published in Diabetes & Vascular Disease Research, Gore and McGuire17 listed 14 Phase III and Phase IV cardiovascular outcomes trials addressing the cardiovascular safety and efficacy of drugs for T2DM. Examples include the dipeptidyl peptidase 4 (DPP-4) inhibitors alogliptin, linagliptin, saxagliptin, and sitagliptin; the incretin glucagon-like peptide 1 (GLP-1) receptor agonists dulaglutide, exenatide long-acting release, liraglutide, and lixisenatide; and the sodium glucose co-transporter 2 (SGLT2) inhibitors canagliflozin and empagliflozin. As an instructive example, consider the trial being conducted with saxagliptin.
The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial is currently underway not only to determine that saxagliptin is not
associated with an unacceptable increase in cardiovascular risk, but that it also reduces cardiovascular risk in patients with T2DM.18 At the end of the preapproval clinical development programme a meta-analysis was conducted using pooled data from the Phase II and Phase III trials, which included a total of eight studies involving 3356 subjects who received saxagliptin at various doses and 1251 subjects who received a control treatment (placebo, metformin, up-titrated glyburide, or a thiazolidinedione). The MACE composite endpoint employed included death, myocardial infarction, stroke, and cardiac ischemic events. The relative risk analysis (conducted as a Cox proportional hazard ratio on adjudicated events) was 0.43 (95% CI, 0.23-0.80). Of note is that the upper CI limit, 0.80, is not only below 1.8 and also 1.3, but it is also below unity (represented here as 0.00). This result indicates that there were fewer cardiovascular events in the saxagliptin treatment group than the control treatment group, hence implying not only acceptable cardiovascular safety but also an actual cardiovascular benefit.19 The authors noted the limitations of such an analysis, and stated that “The hypothesis of CV protection with saxagliptin will be tested prospectively in a large randomized clinical outcome trial evaluating saxagliptin compared with standard of care in patients with type 2 diabetes at increased risk for CV events.”19 From both clinical and marketing perspectives, being able to demonstrate cardiovascular benefit is a very powerful advantage. The SAVOR-TIMI 53 trial is therefore statistically powered to be large enough to be able to determine whether saxagliptin can indeed reduce the risk of cardiovascular events: it intends to enroll 16,500 subjects. While acceptable safety of one drug in a class should not generally be regarded as ‘evidence’ that subsequent drugs in that class will also be acceptably safe, it is of interest here that meta-analyses conducted for other DPP-4 inhibitors have also not detected a cardiovascular safety signal. Cardiovascular outcomes studies are ongoing for alogliptin, linagliptin, and sitagliptin. In conjunction with SAVOR-TIMI 53, these trials will provide a very large amount of clinical outcome data for this class of drug that will address questions of both cardiovascular safety and
cardiovascular benefit.
Concluding Comments
The increasing global prevalence of T2DM requires continued development of new antidiabetic drugs. One aspect of such development is the prospective this topic with the intent of providing prescribing physicians, and all allied health professionals, with a fundamental understanding of the methodology required to provide compelling evidence of the cardiovascular safety of new antidiabetic drugs for the treatment of this disease. ■
References
1. Turner JR, Strumph P. The moral imperative of improving patient adherence to pharmacotherapy for cardiodiabesity, Part I: A focus on type 2 diabetes mellitus. Journal for Patient Compliance. 2012;2(1):32-36.
2. Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice. 2011;94:311-321.
3. American Diabetes Association. http://www.diabetes.org/diabetesbasics/diabetes-statistics. (Accessed 15th October 2012)
4. Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001; 344:1343-1350.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002; 346:393-403
6. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf (accessed 15th October 2012)
7. Turner JR. The 50th Anniversary of the Kefauver-Harris Amendments:Efficacy Assessment and the Randomized Clinical Trial. Journal of Clinical Hypertension, in press. DOI: 10.1111/jch12012.
8. FDA. Guidance for Industry. Diabetes Mellitus—Evaluating cardiovascular risk in new antidiabetic therapies to treat
type 2 diabetes. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/
ucm071627.pdf, December 2008. (accessed 15th October 2012)
9. FDA. Guidance for Industry. Diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/
UCM071624.pdf. February 2008. (accessed 15th October 2012)
10. Summary Minutes of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee, July 1 - 2, 2008. http://www.fda.gov/ohrms/dockets/ac/08/minutes/2008-4368m-Final.pdf. (accessed 15th October 2012)
11. Caveney E, Turner JR. Regulatory landscapes for future antidiabetic drug development (Part I): FDA guidance on assessment of cardiovascularrisks. Journal for Clinical Studies. 2010. January issue, 34-36.
12. Turner JR, Strumph P. FDA and EMA Actions Regarding the Cardiovascular Safety of Drugs for Type 2 Diabetes Mellitus, 2007-2012: An Overview of Respective Regulatory Landscapes. Journal for Clinical Studies. 2012:(3):22-24.
13. Turner JR, Somaratne R, Cabell CH, Tyner CA. Centralized endpoint adjudication in cardiovascular outcomes studies: Composite endpoints, risk ratios, and clinical endpoint committees. Journal for Clinical Studies. 2011;3:46-49.
14. Turner JR. Integrated cardiovascular safety: Employing a threecomponent risk exclusion model in the assessment of investigational drugs. Applied Clinical Trials. 2010;19(6):76-79.
15. Turner JR, 2012, Key Statistical Concepts in Clinical Trials for Pharma. New York: Springer.
16. Joffe HV, Parks MH, Temple R. Impact of cardiovascular outcomes on the development and approval of medications for the treatment of diabetes mellitus. Reviews in Endocrine and Metabolic Disorders. 2010;11(1):21-30.
17. Gore MO, McGuire DK. Drugs for type 2 diabetes mellitus: The imperative for cardiovascular outcome assessment. Diabetes & Vascular Disease Research. 2012;9(2):85-88.
18. Cobble ME, Frederich R. Saxagliptin for the treatment of type 2 diabetes: Assessing cardiovascular data. Cardiovascular Diabetology. 2012;11:6.
19. Frederich R, Alexander JH, Fiedorek FT, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgraduate Medicine. 2010;122(3):16-27.
A Review of Travel-associated Diarrhoeal Illnesses
Barry Dyer MB, BS
Manager, Medical Information & Analysis. International SOS Assistance (UK) Ltd, Chiswick Park, Building 4, 566 Chiswick High Road, London, England W4 5YA, United Kingdom
Barry graduated from King’s College School of Medicine and Dentistry in 1998 and worked in the NHS for seven years, primarily in emergency medicine and anaesthetics. He joined International SOS in 2005 and supported government organisations and international travellers in accessing healthcare around the world. He is currently studying for an MSc in Health Informatics at the University of Edinburgh.
cite as:
Dyer B. A review of Travel- associated Diarrhoeal Illnesses. The Physician
2012 1(1) 40-42
Introduction
Travellers’ diarrhoea is the most common travel-related illness. It usually occurs within the first week away from home, and affects between 10 and 60 per cent of all those who go abroad.1 Travellers are especially likely to become ill at high-risk destinations such as low-income nations in Latin America, Africa, Asia and the Middle East.
Signs and Symptoms
Traveller’s diarrhoea (TD) is defined as the passage of at least three loose or watery stools in 24 hours. This can be accompanied by abdominal cramps, nausea and fever. Vomiting may occur less commonly, and bloodstained stools or mucous are unusual. Symptoms are frequently self-limiting but may persist long after return.2
Aetiology
Travellers’ diarrhoea can be caused by a multitude of organisms: viruses, bacteria and parasites. Most travel-associated diarrhoea is caused by bacterial contamination including ETEC (enterotoxigenic E. coli), Salmonella, Shigella and Campylobacter species. Norovirus is often implicated in large-scale outbreaks on board vessels and in destinations
that would otherwise be deemed low-risk.3 Viruses, such as rotavirus and clacivirus, and parasites such as protozoa
(Cryptosporidium, Giardia or Entameba histolytica) are other common causes. Cyclospora particularly impacts travellers visiting Nepal.4 Although reported frequently in international media, cholera is an infrequent cause of travellers’ diarrhoea 5 as most tourist destinations do not experience cholera outbreaks. However, those who travel to visit friends and relatives (“VFR”) and international aid workers remain at risk.
Investigation
Investigations should be reserved for prolonged diarrhoeal episodes or when stools are bloodstained. This is initially directed at stool culture for microscopy to examine ova, cysts or parasites and to establish sensitivities of any cultured organisms.
Treatment
Most cases of travellers’ diarrhoea will resolve spontaneously within a day or two. Guidance is directed at replacing fluids lost by continuously sipping clear fluids such as water, soft drinks or weak tea. Patients should be directed to avoid dairy products, alcohol and coffee as these can compound symptoms and worsen dehydration. The priority in treating acute diarrhoea is the prevention or reversal of fluid and electrolyte depletion. The extremes of age are particularly susceptible to dehydration. Parents should be advised to seek medical advice early if children are affected.
Oral rehydration preparations are widely available in some countries, but availability does vary. For example, Mexico does not permit the sale of oral rehydration salts, only pre-mixed oral rehydration solution. It is imperative to ensure that any water used to reconstitute oral rehydration salts is safe for human consumption. Occasionally dehydration will require intravenous fluids, especially if there has been significant vomiting or extreme diarrhoea. Travellers should seek treatment with reputable medical facilities that provide quality pharmaceutical goods and adhere to international standards of hygiene.
Medications
Two main types of medications are used to treat travellers’ diarrhoea.
Drugs to slow the diarrhoea
Antimotility drugs
Loperamide
For self-treatment in adults, the recommended initial dose is 4mg after a loose stool, followed by 2mg after each unformed stool. The dose should not exceed 8mg in a 24-hour period. It should not be used if there is a high fever or blood in the stool, or in young children. Patients should be advised to seek medical attention if no better in 48 hours.
Loperamide can be used in conjunction with antibiotic treatment.
Antispasmodics
Hyoscine Butylbromide and others are occasionally of value in symptomatic relief, but should not be used for primary treatment.
Antibiotics
Initial empiric treatment of TD is with an antibiotic known to be active against the most prevalent enteropathogens in the region of travel, and include:
Ciprofloxacin
• 500mg bd for three days. (Single dose therapy can be used.) Cannot be given to children.
Azithromycin
• Adults: 1000mg as a single dose. Safe for children – dosage 10mg/kg per day for three days.
Rifaximin
• 200mg tds for three days. Should not be used if the patient has a fever or blood in the stool as it is ineffective against invasive causes of TD. UK prescribing guidelines do not permit administration in children. For travel to locations with a high risk of TD, it may be advisable to prescribe a standby treatment course of antibiotics for the traveller to carry with
them.6
Prevention
All travellers should be advised to be careful in their selection of food and water. The following guidelines can be given to travellers to developing countries:
Selecting Safe Food
• Always wash your hands with soap before eating, or use a hand sanitising gel/lotion.
• Select food that is thoroughly cooked while fresh and served very hot, since heat usually kills bacteria.
• Avoid undercooked or raw meat, fish or shellfish, even if they are the local delicacy. These are potentially a major source of infection.
• Avoid food sold by street vendors and establishments that don’t have access to safe water for washing produce and utensils.
• Only eat raw fruit you have peeled yourself (oranges, bananas, mangos, avocados, etc).
• Avoid salad and raw vegetables in restaurants. Only eat raw vegetables if they were washed well with safe water.
• Avoid food that has been left unrefrigerated for more than two to four hours, especially if it was kept warm. Food that has been prepared hours (or days!) ago is more likely to be contaminated than freshly cooked food.
• Only drink pasteurised cow, sheep or goat milk. Avoid dairy products (such as ice cream, butter and cheese) if you do not know if they have been made from pasteurised milk.
Avoiding Contaminated Water
Where there is a risk that the tap water may be contaminated:
• Bottled water and drinks are normally safe, especially carbonated drinks. Look for an intact seal.
• The outside of cans or bottles may be contaminated, especially if they were stored in ice. Clean and dry bottles and cans before drinking from them or pouring the liquid into a glass.
• Remember that ice may have been made from contaminated water, or contaminated afterwards through handling, and therefore may not be safe.
• Use safe water for brushing teeth and for washing raw vegetables and salad.
• Don’t drink the water from open wells or rivers. If bottled water is not available, the following alternative means of
sterilisation can be used:
• Boiling water is a reliable method for making it safe to drink. Bring water to a full boil for one minute and allow it to cool to room temperature (longer if at altitude).
• Disinfectants
• Iodine is very effective: Four drops of two per cent tincture of iodine should be added to each litre of water and left for 15 minutes. Avoid using iodine for prolonged periods (longer than six weeks).
• Sterotabs and Puritabs: These are chlorine-based and are less effective against some infectious agents, including amoebic cysts.
• In an emergency, use household bleach: use two to four drops per litre of clear water and leave for 15 minutes. This is safe and effective but will leave water tasting of chlorine. (Disinfecting can be ineffective if the water is visibly cloudy.)
Portable Water Filters
There are several types of water filters. Each provides various degrees of protection against microbes:
• Reverse-osmosis filters provide protection against viruses, bacteria and parasites. They are expensive and large, and the small pores of these filters are easily blocked by muddy or cloudy water.
• Microstrainer filters can remove bacteria and parasites from drinking water, but they do not remove viruses. To remove viruses, travellers using microstrainer filters should also disinfect the water with iodine or chlorine after filtration.
• Filters with iodine-impregnated resins are most effective against bacteria. The iodine in these filters can also kill some viruses, but the contact time does not allow the killing of parasites such as Cyptosporidium and, in cold water, Giardia. It is important to maintain and replace the cartridges as specified by the manufacturer’s instructions.
Vaccination
There are vaccines available for some faecal-orally transmitted organisms such as Salmonella typhi, hepatitis A, and Vibrio cholerae. Vaccinated travellers may still develop diarrhoea caused by other organisms. The Medicines and Healthcare products Regulatory Agency advised that the availability of some typhoid-containing vaccines became limited in early October 2012 following a recall of Sanofi-manufactured vaccines.7,8 Seek MHRA recommendations in the event of vaccine shortages. The oral B-subunit cholera vaccine Dukoral® gives good protection against cholera and halves the risk of developing traveller’s diarrhoea caused by ETEC (enterotoxigenic E. coli). It is available in an increasing number of countries, including Canada and the UK, among other European nations.9 However, travellers who use this vaccine should also carry self-treatment remedies in the event that they develop diarrhoea that is not caused by ETEC.10
Summary
An ounce of prevention is worth a pound of cure, and all travellers should be educated in the selection of safe food and water. Travellers to high-risk destinations or with high-risk itineraries should consider pre-departure vaccination against typhoid and hepatitis A. In addition, carrying a course of rehydration preparation, antimotility agent, and treatment antibiotics is advisable. ■
References
1. Dupont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Alimentary Pharmacology & Therapeutics.303:187–96.
2. Hill DR. Occurrence and self-treatment of diarrhea in a large cohortof Americans traveling to developing countries. Am J Trop Med Hyg. 2000 May 1;625 :585–9.
3. Apelt N, Hartberger C, Campe H, Löscher T. The Prevalence of Norovirus in returning international travelers with diarrhea. BMC Infectious Diseases. 2010 May 25;10(1):131.
4. Pawlowski SW, Warren CA, Guerrant R. Diagnosis and Treatment of Acute or Persistent Diarrhea. Gastroenterology. 2009 May;1366 :1874–86.
5. European Centre for Disease Control & Prevention. Annual epidemiological report on communicable diseases in Europe 2010. Stockholm: ECDC; 2010.
6. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008 Oct 6;337(oct06 2):a1746–a1746.
7. Medicines and Healthcare products Regulatory Agency (MHRA) www mhra gov uk. Press release: Typhoid vaccine batches recalled by manufacturer Sanofi [Internet]. [cited 2012 Oct 18];Available from: http://www.mhra.gov.uk/NewsCentre/Pressreleases/CON192154
8. Health Protection Agency. Typhoid fever vaccine recalled [Internet]. [cited 2012 Oct 18];Available from: http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1317136430366?p=1317132140479
9. World Health Organization. Cholera vaccines: WHO position paper. Weekly epidemiological record. 2010 Mar 26;8513:117–28.
10. Jelinek T, Kollaritsch H. Vaccination with Dukoral against travelers’ diarrhea (ETEC) and cholera. Expert Rev Vaccines. 2008 Jul;75:561–7.
Perennial Rhinitis
Glenis Scadding
Consultant Allergist & Rhinologist,
RNTNE Hospital, London
cite as:
Scadding G. Perennial Rhinitis. The Physician
2012 1(1):44-46
Rhinitis - Definition and Classifications
Rhinitis means nasal inflammation, and sufferers have two or more of the symptoms of running, itching, sneezing and blocking. It has traditionally been classified as seasonal or perennial, or both. Most people are familiar with seasonal allergic rhinitis, or hay fever, as it is known in the UK. In this the symptoms occur when seasonal pollens enter the nose of people who are allergic to them - grass pollen is the commonest, and the problem is seen in the summer. Perennial rhinitis occurs throughout the year. It is sometimes allergic in nature and is then caused by allergens which are present all year, such as pets, house dust mites, moulds and occupational allergens.
The ARIA (Allergic Rhinitis and its Impact on Asthma)
classification of rhinitis uses a different approach: symptoms are either intermittent
(less than four days a week ,or less than four weeks at a time), or persistent, in which case they exceed four days at a time for over four weeks. Persistent does not equate to perennial since perennial sufferers are not always troubled to that extent; similarly seasonal allergic rhinitis is not necessarily intermittent , but can be persistent, particularly at the height of the season.
The ARIA classification also introduced a system for assessing AR severity based on the impairment of four health-related quality of life (HRQL) items: sleep, daily activities/sport and leisure, work or school performance, and troublesome symptoms. According to the ARIA workshop group, AR is mild when there is no impairment of any of these
items, while it is moderate/severe when one or more of these items are impaired 1. Thus perennial rhinitis can be intermittent or persistent, and mild or moderate to severe.
Prevalence and Co-morbidities
Rhinitis is common: Allergic rhinitis affects 10-30% of the adult population. Approximately 80% of individuals diagnosed as having AR develop symptoms before the age of 20. Older children have a higher prevalence of AR than younger ones, with a peak occurring in children aged 13-14. While boys are more likely than girls to have AR, this tendency switches in puberty so that equal numbers of adults are affected. A postal survey within the UK in 1991 reported the prevalence of all forms of rhinitis (excluding infectious) as 24%, with 30% seasonal, 13% perennial and 57% mixed. However, a more recent study puts the total nearer 30% of the population.
Symptoms of allergic rhinitis
(rhinorrhea, nasal congestion, itch and sneezing) impair the performance of daily activities, and reduce sleep quality, cognitive function, school and work attendance, and productivity. Severity, more than the persistence of symptoms, is the factor most affecting quality of life and school performance. Rhinitis generates an important socioeconomic burden. AR is also closely linked to other inflammatory diseases affecting the respiratory mucous membranes, such as asthma, rhinosinusitis, otitis media with effusion, and allergic conjunctivitis. An association between asthma and allergic rhinitis is noted in epidemiological, experimental, functional and clinical studies, including evidence of significant improvement in outcomes when the upper airway is appropriately treated. Around one-third of rhinitis patients also have asthma – but those who have not yet developed asthma have a risk of so doing some three times higher than non-rhinitics. This is true for both allergic and non-allergic rhinitis. The mean time between the development of asthma in rhinitics is two years, allowing a window of opportunity for possible prevention of progression. Most asthma patients also suffer from rhinitis, whether atopic or non-atopic. Atopy is the abnormal tendency to develop specific IgE in response to innocuous and ubiquitous environment allergens. Atopic diseases include allergic rhinoconjunctivitis, asthma, atopic dermatitis and
food allergies, and these run in families. Atopy has been linked to multiple genetic loci, including those on chromosomes 2, 5, 6, 7, 11, 13, 16 and 20.
Development of atopic disease occurs in 13% with no family history, 29% if one parent or sibling was atopic, 50% if both parents were atopic and 72% if they shared the same atopic manifestation. Other risk factors for allergic rhinitis include being non-Caucasian, firstborn, or of high socioeconomic status, environmental pollution, birth during a pollen season, late entry into daycare, heavy maternal smoking, indoor allergen exposure, serum IgE level >100 IU/mL before age six, the presence of positive skin-prick tests, and early introduction of foods or formula. Multiple studies have also found that early environmental exposure to various infectious agents such as mycobacteria, hepatitis A, Toxoplasma gondii and lipopolysaccharide (found in Gram negative bacteria), protects against development of atopy.
Most asthma exacerbations (80% in children and over 60% in adults) are related to upper respiratory viral infections – primarily due to rhinoviruses. Allergic children suffer more from colds, which tend to last longer and to be more complicated. In both mite-induced and pollen-induced rhinitis minimal persistent inflammation (MPI), a low-grade inflammatory infiltration in the mucosa unaccompanied by obvious symptoms, has been demonstrated with weak and persistent expression of intercellular adhesion molecule-1 (ICAM-1), the major receptor for human rhinoviruses. This over-expression in asymptomatic allergic subjects is important since synergy occurs between allergic and infective inflammation. The combination of a rhinoviral cold in a sensitised asthmatic child exposed to relevant allergen gives a nearly 20-fold risk of hospitalisation for asthma. Therefore perennial rhinitis demands accurate diagnosis and effective treatment.
Diagnosis
Rhinitis is often undiagnosed, misdiagnosed and / or mistreated. The patient’s history is most important. In seasonal rhinitis the nasal symptoms are usually obvious, with immediate sneezing, itching and running related to mast cell degranulation in relation to allergen exposure (early phase response). Conjunctivitis and itchy eyes accompany nasal symptoms in 70%, aiding the differential diagnosis from the common cold. In perennial rhinitis the predominant symptom is often a blocked nose; especially when the problem is persistent, rhinorrhoea can be predominantly posterior, and the relationship to allergen hidden. This is because the late phase response to allergen predominates and involves inflammation with eosinophils, a cardinal feature (Figure 1). Conjunctivitis is less likely (under 50%). Inflammation may extend beyond the nasal cavity to involve the sinus linings, when it is known as rhinosinusitis.
Symptoms
can include nasal blockage, rhinorrhoea, facial pain, headache and reduced sense of smell. If a history of exacerbation and remission in response to possible allergens (pets, house dust mite, moulds, work) is not sought and corresponding skin-prick or blood IgE tests not arranged, then the allergic nature of the problem can be missed. Even among those with negative skin-prick tests there is a population of subjects with local allergic rhinitis who respond to nasal allergen challenge. Conversely positive skin-prick tests to allergens such as house dust mites can be found in asymptomatic
people (sensitisation without clinical disease), thus a history of reaction upon exposure and improvement on avoidance is needed. The differential diagnosis of perennial rhinitis is wide, and includes serious diseases such as Wegener’s granulomatosis in adults and reduced immunity in children (primary ciliary dyskinesia, cystic fibrosis). All subjects
need careful nasal, full ENT, and chest examinations. Those with unusual symptoms such as bleeding, unilateral discharge, nasal collapse or septal perforation and/or systemic illness need full systematic review and screening blood tests. Those with non-allergic disease can be subdivided into inflammatory and non-inflammatory forms by nasal smears. In nonallergic eosinophilic rhinitis, aspirin sensitivity should be considered, especially if nasal polyps and asthma are present.
Treatment
For allergic rhinitis the ARIA document suggests basic underlying treatment with allergen and pollutant avoidance, plus consideration of nasal douching with saline. Sprays such as Sterimar are a convenient aid to concordance with this recommendation, and can be used even in small children when symptoms are troublesome or after exposure to allergens or pollutants. Saline douching is also effective in chronic rhinosinusitis. Antihistamines are useful for mild intermittent symptoms; more Figure 1.
The allergic response with an early phase related to immediate mast cell degranulation upon allergen exposure and IgE cross-linking by allergen and a late phase, often seen with chronic or very high-dose allergen exposure, where inflammation predominates. Symptoms are rapid and obvious in the early phase, but chronic obstruction, hyper-reactivity and reduced olfaction characterise the late phase
From Stephen Durham, Imperial College, London, with thanks.
Severe disease requires pharmacotherapy, with intranasal corticosteroids the treatment of choice, being demonstrably more effective than antihistamines or montelukast in meta-analyses of clinical trials. Modern molecules such as mometasone furoate, fluticasone propionate and furoate have proved safe in use over a year in children. Combinations of therapy are used when one fails: recent trials of a spray containing fluticasone propionate plus azelastine show better efficacy than either alone.
Some 20% of AR sufferers remain uncontrolled by guideline-directed pharmacotherapy – these should be referred to a specialist for consideration for immunotherapy where there is a clear allergen driver of symptoms. Nasal allergen challenge may be needed to ascertain the relationship of perennial rhinitis to allergen exposure before embarking on expensive allergen-specific treatment. Immunotherapy is now available by both subcutaneous and sublingual routes, the latter being safer and more applicable to children, sadly with restricted availability of allergens as yet, since alteration of disease progression is a necessary outcome imposed by European regulatory authorities. Perennial allergens such as house dust mite and cat are at present in clinical trials.
In summary, perennial rhinitis involves a spectrum of disease from mild, intermittent forms which are likely to relate to occasional allergen exposure and can be treated by allergen identification and avoidance, backed up by saline douching; to severe, persistent disease which needs accurate diagnosis and specific treatment, but where saline douching may still play a role in therapy. ■
Further reading
1, Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147-334.
2, Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, Ryan D, Walker SM, Clark AT, Dixon TA, Jolles SR, Siddique N, Cullinan P, Howarth PH, Nasser SM; British Society for Allergy and Clinical Immunology.BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008 Jan;38(1):19-42.
3, Greiner A, Hellings P, Rotiroti G, Scadding GK. Allergic Rhinitis, Lancet 2011, July 21st,epub ahead of print.
4, Hermelingmeier KE, Weber RK Helmich M et al. Nasal irrigation as an adjunctive treatment in allergic rhinitis: A systematic review and meta-analysis. Am J Rhinol. 26,5, e119-e125.
Resurgence of Vitamin D Deficiency Rickets
M Zulf Mughal
Consultant in Paediatric Bone Disorders & Honorary Senior Lecturer in Child Health
Royal Manchester Children’s Hospital, Central Manchester University Hospitals NHS Foundation Trust, Oxford Road
Manchester M13 9WL
Cite as:
Mughal MZ. Resurgence of Vitamin D Deficiency Rickets.
The Physician
2012 1(1): 48-51
Introduction
Rickets is a disorder of the growing child arising from disorders that result in impaired apoptosis of hypertrophic cells and mineralization of the growth plate. Rickets arising from deficiency of vitamin D remains an important health problem in many developing and developed countries. In this article I shall discuss factors that lead to vitamin D deficiency rickets. Diagnosis, treatment and prevention of vitamin D deficiency rickets will also be discussed.
What Is Rickets?
Rickets is a disease of the growing child in which there is failure of mineralization the growth plate and osteoid matrix. The orderly differentiation of the growth plate is regulated by a number of growth and transcription factors. Cartilage cells in the ‘resting zone’ of the growth plate, adjacent to the epiphysis, mature into chondrocytes. These chondrocytes become organized into columns, aligning themselves along the longitudinal axis and undergo hypertrophy. The terminally
differentiated hypertrophic chondrocytes, undergo vascular invasion, apoptosis and mineralization. The scaffold left behind by apoptotic chondrocytes is turned into to primary spongiosa by invading osteoclasts. Low serum phosphate is responsible for the reduced apoptosis of hypertrophic chondrocytes and development of rachitic changes 1,2. In vitamin D deficiency low serum phosphate concentration arises from elevated serum parathyroid hormone (PTH) levels, which in-turn causes in renal phosphate wastage from proximal renal tubules. The accumulation of hypertrophic chondrocytes in the growth plate, secondary to hypophosphatemia gives rise to clinical signs of rickets, such as the hypertrophy of the costochondral junctions and swelling of ends of long bones. It also results in the radiological signs of widening of metaphyses
Sources & Metabolism Of Vitamain D
Vitamin D exists in two forms: vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). In humans over 90% of vitamin D comes from the photo conversion of 7-dehydrocholesterol in the skin to cholecalciferol by solar ultraviolet radiation (UVB; 290-320 nm). Vitamin D3 is derived from natural dietary sources such as oily fish, eggs & liver and fortified foods makes a small contribution to body sores of vitamin D. Ergocalciferol or vitamin D2 is derived from plant and fungal sources, or produced commercially by irradiation of yeasts. The two forms undergo identical metabolism and will be referred to as vitamin D when it is not necessary to distinguish between them. Vitamin D is metabolized to 25-hydroxyvitamin D 25(OH)D by a number of cytochrome P450 (CYP) enzymes. A rare case of rickets due to CYP2R1 mutation suggests that this might be an important CYP enzyme responsible for synthesis of 25(OH)D 3,4. Serum 25(OH)D is the major circulating form of vitamin D and it’s serum concentration is used to determine an individual’s vitamin D status. Circulating 25(OH)D is hydroxylated by the 1 hydroxylase enzyme (CYP27B1) in the kidney to 1,25 dihydroxyvitamin D (1,25(OH)2D), the biologically active metabolite of vitamin D. The activity of, CYP27B1 is stimulated by PTH and low serum concentration of calcium and phosphate. It’s activity is inhibited by the fibroblast growth factor 23 (FGF23), a hormone that is produced by osteocytes which plays an important role in phosphate homeostasis (see below). 1,25 dihydroxyvitamin D acts on its nuclear receptor, the vitamin D receptor (VDR) in intestinal cells to promote gastrointestinal absorption calcium & phosphorus. 1,25(OH)2D is also important for calcium homeostasis. When dietary calcium intake or serum ionised calcium concentration is low, 1,25(OH)2D interacts with the VDR in osteoblasts to induce the expression of the plasma membrane protein receptor activator of NF-κB ligand (RANKL). The RANKL binds to RANK on preosteoclasts causing them to mature to osteoclasts, which in turn cause bone resorption, releasing calcium & phosphorous into the circulation. Both 25(OH)D and 1,25(OH)2D are catabolized by 24-hydroxylase (CYP24A1) to inactive metabolites, 24,25-dihydroxyvitamin D and calcitroic acid respectively.
Vitamain D Deficiency Rickets
Vitamin D deficiency remains an important cause of rickets in many parts of the world 5. In spite of abundance sunshine, vitamin D deficiency rickets in not uncommon in Middle Eastern countries 6. 7.8. 9 10. There appears to be
resurgence among children of ethnic minorities living in Europe and North America 11, 12, 13. It is a disorder of the growing child and is therefore manifests during infancy (usually < 18 months of age) and during the adolescent growth spurt. Detailed discussion of factors responsible for vitamin D deficiency rickets is beyond the scope of this review but include:
a) Residence at latitudes where the sun is too low in the sky during winter months, for example among < 1yr old infants in Northern territories of Canada 13.
b) Atmospheric pollution, which limits UVB reaching the ground level 14.
c) Wearing of clothing which cover most of the skin surface area & because of voluntary sunshine avoidance for religious and cultural reasons 15 ,16.
d) Maternal vitamin D status during pregnancy. Vitamin D deficiency in pregnant women increases the risk for rickets in their offspring. 17.
e) Skin pigmentation; melanin absorbs ultraviolet B radiation thus diminishing cutaneous vitamin D synthesis 18.
f) Rachitogenic role of vegetarian diets 19.
g) Low calcium diet, which induces secondary hyperparathyroidism. High serum PTH leads to increased synthesis of 1,25(OH)2D, which is known to degrade 25(OH)D to inactive 24,25(OH)2D, thereby depleting body stores of vitamin D 20,21.
h) Prolonged and exclusive breast feeding without vitamin D supplements 22; human milk contains only about a 40 i.u (1 µg) per liter.
i) Genetic and ethnic differences in vitamin D metabolism 23,24. Clinical features vary with the severity and the age of onset of rickets. Florid skeletal deformities are more common in infancy. Infants with rickets usually develop deformities of their weight bearing limbs. A crawling child develops deformities in the forearms, whereas a walking toddler develops bow legs (genu varum) or knocks knees (genu valgum). Other features of rickets include growth retardation, frontal bossing of the skull, swelling of wrists, knees, and ankles. ‘Rachitic rosary’ arises due to expansion of the costo chondral junctions, and an inward diaphragmatic pull of soft rib cage gives rise to Harrison’s sulcus (groove). Dentition may be delayed and development of tooth enamel impaired. Irritability, considered to be secondary to bone pain, is a common feature in rachitic infants. Muscle weakness associated with vitamin D deficiency leads to hypotonia and delay of the motor development. Adolescents with rickets usually present with vague symptoms such as aches and pains in lower limbs, which are precipitated by walking or playing games. They also complain of muscle weakness and the proximal
myopathy may cause difficulty in climbing stairs. Frequently musculoskeletal symptoms in these youngsters are attributed to ‘growing pains’ and are inappropriately treated with analgesics. Florid signs of rickets are rare in adolescents. Deformities such as bowlegs and knock knees may develop with long standing vitamin D deficiency. Pelvic deformities that develop during adolescence can later lead to obstructed labor. Most children with vitamin D deficiency rickets have serum 25(OH)D concentrations <10 ng/ml, and usually < 5 ng/ml. However, serum 25(OH) D concentrations may not be markedly reduced in overtly rachitic children who have low dietary calcium intake. In a study from Connecticut, USA, 50% of infants with clinical, biochemical and radiological signs of rickets had serum 25(OH)D concentrations >20 ng/ml 25. In the early stages of vitamin D deficiency, serum calcium concentration is low with a normal serum phosphate concentration. Hypocalcemia leads to secondary hyperparathyroidism, which in turn results in an increase in serum 1,25(OH)2D concentration, normalization of serum calcium concentration and a decrease in serum phosphate concentration. At this stage serum 25(OH)D concentration is low and the concentration of 1,25(OH)2D is normal or high. This biochemical state is maintained at the expense of the resorptive action of PTH on bone. Long standing vitamin D deficiency eventually leads to recurrence of hypocalcaemia. Serum alkaline phosphatase activity is
raised above the upper limits of normal for the age. Radiological signs are seen at rapidly growing ends of long bones, for example at the distal rather than the proximal end of the femur. The earliest radiological sign of rickets is the loss of the crisp line, produced by the zone of provisional calcification at the interface of the epiphyseal growth plate and metaphyses of long bones, is lost. This zone becomes frayed or ‘brush like’, and in more advanced stages of rickets it becomes concave or “cup shaped”. The metaphyseal area also becomes wider than normal [Figure 1]. These metaphyseal changes tend to be more marked in toddlers rather than in adolescents with rickets. Radiological features of secondary
hyperparthyrodism include generalized osteopaenia, subperiosteal bone resorption and periosteal reaction along diaphyes.
Treatment & Prevention
There are numerous regimens for treatment of vitamin D rickets 26, however, it is fairly straightforward and involves administration of vitamin D2 or D3 in therapeutic doses (1500 6000 i.u./daily) until there is normalization of biochemistry. Usually treatment is necessary for 2 to 3 months; it important to provide calcium supplements in those with inadequate dietary calcium intake. Some advocate use of a single-day large oral dose of vitamin D or ‘Stoss therapy’, particularly in those with poor compliance 27 However, such regimens can be associated with increased risk of hypercalcemia 28. Improvement in clinical symptoms, such as aches and pains occur within 2 weeks, and in toddlers, the disappearance of swelling of the distal ends of long bones (metaphyses) occurs usually by 6 months. In this group, full correction of bowed legs or knock-knees can take up to 2 years, but the adolescents are usually left with residual skeletal deformities that require surgical correction.
Vitamin D supplementation has been shown to be safe and effective way of reducing vitamin D deficiency rickets in vulnerable infants 29. A nationwide program of providing free vitamin D drops (400 i.u. daily) to newborns and infants reduced the prevalence of rickets from 6% in 1998 to 0.1% in 2008 in children less than 3 years of age 30. ■
References
1. Sabbagh Y., Carpenter T. O., Demay M. B. (2005) Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl. Acad. Sci. U.S.A. 102, 9637–9642
2. Tiosano D & Hochberg Z Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab. 2009;27(4):392-401.
3. Casella, S.J., Reiner, B.J., Chen, T.C., Holick, M.F. 1994. A possible defect in 25-hydroxylation as a cause of rickets. J. Pediatr. 124:929–932.
4. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (2004) Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 101:7711–7715.
5. Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16
6. Al-Atawi MS, Al-Alwan IA, Al-Mutair AN, Tamim HM, Al-Jurayyan NA. Epidemiology of nutritional rickets in children. Saudi J Kidney Dis Transpl. 2009 Mar;20(2):260-5.
7. Abdullah MA, Salhi HS, Bakry LA, Okamoto E, Abomelha AM, Stevens B, Mousa FM. Adolescent rickets in Saudi Arabia: a rich and sunny country. J Pediatr Endocrinol Metab. 2002;15(7):1017-25
8. Bener A, Al-Ali M, Hoffmann GF. High prevalence of vitamin D deficiency in young children in a highly sunny humid country: a global health problem. Minerva Pediatr. 2009;61:15-22.
9. Baroncelli GI, Bereket A, El Kholy M, et al. Rickets in the Middle East: role of environment and genetic predisposition. J Clin Endocrinol Metab. 2008;93(5):1743–1750
10. Rajah J, Thandrayen K, Pettifor JM. Clinical practice: diagnostic approach to the rachitic child. Eur J Pediatr. 2011;170(9):1089-96.
11. Allgrove J. Is nutritional rickets returning? Archives of Disease in Childhood. 2004;89(8):699–701.
12. Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. European Journal of Endocrinology. 2009;160(4):491–497
13. Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. Canadian Medical Association Journal. 2007;177(2):161–166.
14. Agarwal KS, Mughal MZ, Upadhyay P, Berry J, Mawer EB, J.M. P. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Chld. 2002;87:111-113.
15. Das G, Crocombe S, McGrath M, Berry JL, Mughal MZ. Hypovitaminosis D among healthy adolescent girls attending an inner city school. Archives of Disease in Childhood. 2006;91(7):569–572.
16. van der Meer I M, Karamali N S, Boeke A J. et al High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr 2006. 84(2)350–353.353.
17. Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborns of high-risk mothers. Arch Dis Chld Fetal Neonatal Ed. 2007
18. Clemens TL, Adams JS, Henderson SL, Holick MF Increased skin pigmentation reduces the capacity of skin to synthesize vitamin D3. Lancet 1982; 1:74-76.
19. Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr. 2011;14(2):340-6.
20. Clements M R, Johnson L, Fraser D R. A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature 1987. 32562–65.65.
21. A Khadilkar, G Das, M Sayyad, N Sanwalka, D Bhandari, V Khadilkar, M Z Mughal. Letter. Low Calcium intake & Hypovitaminosis D in Adolescent Girls. Archives of Disease in Childhood. 2007;92 (11):1045.
22. Mughal MZ, Salama H, Greenaway T, Laing I, Mawer EB. Florid rickets associated with prolonged breast feeding without vitamin D supplementation. BMJ 1999; 318: 39-40.
23. Awumey EMK, Mitra DA, Hollis BW, Kumar R, Bell NH. Vitamin D metabolism is altered in Asian Indians in the South United States: a Clinical Research Center Study. J Clin Endocrinol Metab. 1998;83:169-173.
24. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, et al. Common genetic determinants of vitamin D insufficiency: a genomewide association study. Lancet. 2010;376:180–188.
25. DeLucia MC, Mitnick ME, Carpenter TO. Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for reexamining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab. 2003;88(8):3539-3545.
26. Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE 2008 Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab 93:2716–2721.
27. Shah BR, Finberg L 1994 Single-dose therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr 125:487-490.
28. Cesur Y, Caksen H, Gündem A, Kirimi E, Odabaş D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab. 2003;16(8):1105-9.
29. Dunnigan M G, Glekin B M, Henderson J B. et al Prevention of rickets in Asian children: assessment of the Glasgow campaign. BMJ 1985. 291(6490)239–242.242.
30. Hatun S, Ozkan B, Bereket A. Vitamin D deficiency and prevention: Turkish experience. Acta Paediatr. 2011;100(9):1195-9.